
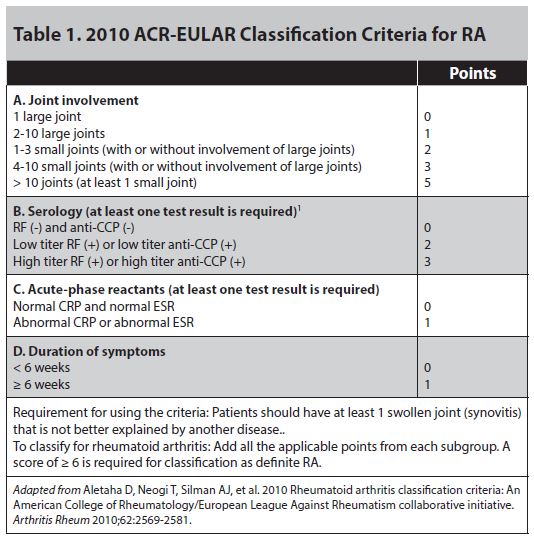
Anti-citrullinated peptide antibodies are a highly disease-specific biomarker with an impact mostly on diagnosis and classification ( 5). TNF inhibitors are generally effective and well-tolerated ( 1, 2) however, up to one-third of patients are primary non-responders, and responses in up to one-third of initial responders abate over time ( 3, 4).Ĭurrently, only a few markers for diagnostic and stratification purposes are used in daily clinical practice in patients with RA. The pro-inflammatory cytokine tumor necrosis factor (TNF) plays a central role in the pathogenesis of RA and is the target of treatment with TNF inhibitors. Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation that, if not treated early and efficiently, causes joint damage. These signatures may support studies of disease pathogenesis, provide candidate markers for response, and non-response to TNF inhibitor treatment, and aid the identification of future therapeutic targets. Expression patterns in a set of functional markers and specific immune cell subsets were distinct in RA patients compared to healthy individuals. Inclusion of the markers p-Akt and CD120b resulted in the correct classification of 18 of 20 RA patients and 17 of 20 healthy donors in regression modeling based on a combined model of basal and TNF-induced signal. All three analysis pipelines identified p-p38, IkBa, p-cJun, p-NFkB, and CD86 in cells of both the innate arm (myeloid dendritic cells and classical monocytes) and the adaptive arm (memory CD4 + T cells) of the immune system as markers for differentiation between RA patients and healthy donors.

The resulting high-dimensional data were analyzed in three independent analysis pipelines, characterized by differences in both data clean-up, identification of cell subsets/clustering and statistical approaches. We employed mass cytometry (CyTOF) with a panel of 13 phenotyping and 10 functional markers to explore signaling in unstimulated and TNF-stimulated peripheral blood mononuclear cells from 20 newly diagnosed, untreated RA patients and 20 healthy donors. The objective of this study was to identify markers in immune cell populations that distinguish RA patients from healthy donors with an emphasis on TNF signaling. Markers for a more exact patient classification and stratification are lacking.
However, the considerable interpatient variability in response to treatment is a challenge. Currently, treatment decisions are primarily based on empirics and economic considerations. The pro-inflammatory cytokine tumor necrosis factor (TNF) plays a central role in the pathogenesis of RA, and TNF inhibitors effectively repress inflammatory activity in RA. RA is a heterogeneous disease with a plethora of treatment options. Rheumatoid arthritis (RA) is a chronic autoimmune, inflammatory disease, characterized by synovitis in small- and medium-sized joints and, if not treated early and efficiently, joint damage, and destruction.


 0 kommentar(er)
0 kommentar(er)
